Nucleic acid medicines are pharmaceutical products produced from the structural components of DNA and RNA, called nucleic acid (oligonucleotide). Through direct action on gene expression, these medicines hold great promise for treating diseases that were formerly difficult to treat. As a pharmaceutical product, they are second only to antibody drugs, and the nucleic acid medicine market is expected to expand in the near future. One way they differ from antibody drugs is that nucleic acid medicines can be synthesized. We develop and distribute NittoPhaseTM high-performance polymer beads for synthesis of nucleic acid medicine (essentially carriers for solid‐phase synthesis), while also providing manufacturing and services to pharmaceutical and biotechnology companies specializing in innovative development of nucleic acid medicine. NITTO DENKO Avecia is at the core of these efforts, which boasts the top share in entrusted manufacturing of nucleic acid medicines.
NITTO DENKO Avecia is a biotechnology company entrusted with nucleic acid medicine synthesis and manufacturing, with two branches in the United States, located in Milford, Massachusetts and Cincinnati, Ohio. To strengthen our business foundation in the promising field of nucleic acid medicine, we acquired and newly established Avecia Biotechnologies, Inc. in February 2011, a company boasting top share in the field of nucleic acid medicine manufacturing.
NITTO DENKO Avecia manufactures a wide range of bulk nucleic acid medicines from the preclinical to commercial facility stages, including antisense, siRNA, aptamer, immunostimulants, miRNA, and decoy molecules. Manufacturing is performed in FDA approved cGMP facilities. It also offers a wide range of services, including analysis method development, process validation, stability testing, radiolabeling, quality control, and pharmaceutical affairs support.
 |
With the completion of the Human Genome Product, the causes of a wide range of diseases as well as pathological mechanisms are increasingly revealed on a genetic level. With these breakthroughs comes the discovery of new methods of treatment.
In advanced disease treatment methods such as gene or nucleic acid therapies, genes or their components are administered into the human body, utilizing genes' own characteristic functions to cure diseases.
Nucleic acids which are chemically synthesized in order to suppress gene or protein activity are generally referred to as nucleic acid medicines.
This includes the mechanism siRNA, a type of RNA interference, for which the Nobel Prize in Physiology or Medicine was awarded in 2006, only eight years after its discovery, attracting great attention as an up and coming new therapy. Our materials that were developed using polymer synthesis technology are now being used in gene and nucleic acid therapies.
 Particles for Nucleic Acid Synthesis |
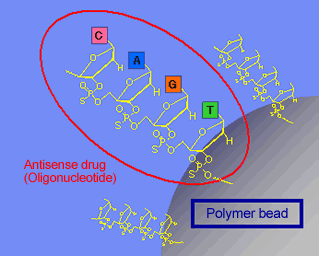 Image of Nucleic Acid Synthesis on Top of or inside the Particle |
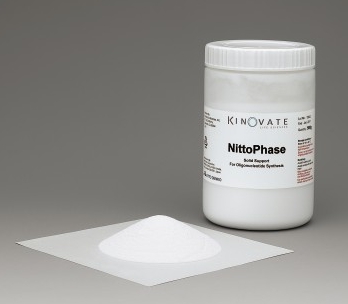 Particles for Nucleic Acid Synthesis Bead:NittoPhase |
NittoPhase is sold exclusively by Kinovate Life Sciences, Inc. worldwide.
Business Hours(Singapore time)08:30 to 17:30 Except Sat, Sun & Public Holidays
TEL +65-6223-8277
Business Hours(Singapore time)08:30 to 17:30
Except Sat, Sun & Public Holidays